How to Calculate Acid Neutralizing Capacity
Acid-neutralizing capacity or ANC in short is a measure for the overall buffering capacity against acidification of a solution eg. Carbonate alkalinity is the acid-neutralizing capacity attributable to carbonate solutes.
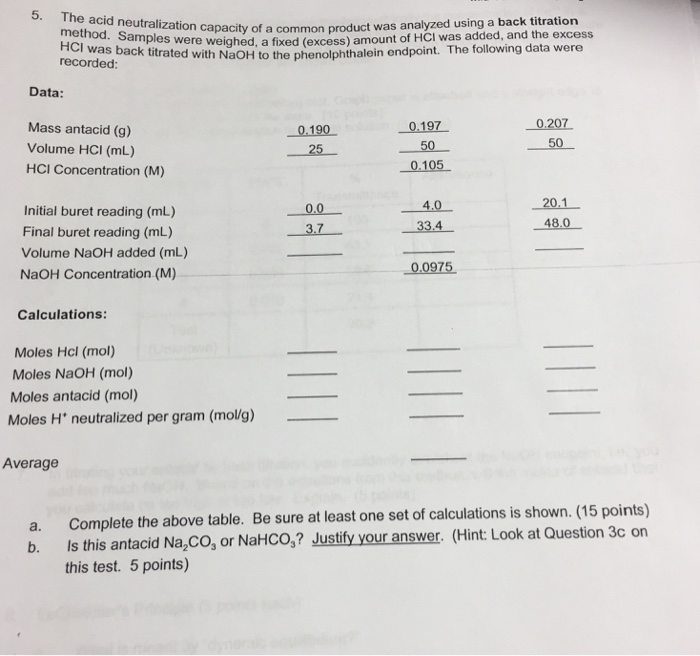
Solved The Acid Neutralization Capacity Of A Common Product Chegg Com
Carbonate alkalinity is the acid-neutralizing capacity attrib-.

. Laboratory Research in Environmental Engineering. NAPP MP A ANC 1 where MP A is the maximum potential acidity formed by the sample ANC is the acid-neutralization capacity of. ANC or Alk is the primary.
Of the net acid-production potential NAPP. Examples of neutralization reaction. If it cant buffer very well the pH may drop quickly with exposure to acid.
Alkalinity should not be confused with pH. Therefore the number of moles of HCl that reacted with the antacid should be equal to the total number of moles of HCl minus the number of moles of excess HCl. This measure of acid-neutralizing capacity is important in figuring out how buffered the water is against sudden changes in pH.
Take this amount and divide by the mass of the sample and you have your acid neutralizing capacity. To nd the number of moles of acid neutralized by the tablet the number of moles of acid neutralized in the titration is subtracted from the moles of acid in the initial solution. In this experiment the acid -neutralizing power of the antacids will be determined by adding a known excess of hydrochloric acid stomach acid and back-titrating the excess acid with standardized sodium hydroxide.
The relationship between alkalinity pH organic acid concentration and inorganic carbon content. ANC is the acid-neutralizing capacity of solutes plus particulates in an unfiltered water sample reported in milliequivalents or microequivalents per liter. Total mEq 30 N HCl V NaOH N NaOH in which N HCl and N NaOH are the normalities of the hydrochloric acid VS and the sodium hydroxide VS respectively.
Completely neutralize the acid. And V NaOH is the volume of. To determine the amount of base in an actual tablet ideally you would dissolve it in water and titrate with acid.
Titrate the excess HCl with 05 N NaOH to attain a stable pH of 35. CALIBRATION AND 662 STANDARDIZATION Alkalinity and Acid Neutralizing CapacityUS. Dilute with carbon dioxide-free DIW to the 1-L mark.
The solution will be titrated with base of known concentration to determine the amount of acid not neutralized by the tablet. Alkalinity is a measure of the capacity of water or any solution to neutralize or buffer acids. Calculate the number of meq of acid consumed and express the result in terms of.
The reaction of acid and base which produces salt and water is called a neutralization reaction. Acid neutralizing capacity per gram of antacid. Using data from Part A Calculate the molar concnetration of the dilute antacid-HCL solution and of the original antacid-HCL solution for each trial Than calculate the average concentration of the original anacid-HCL solution.
Et s VN V 19 pH Measurements The pH can be measured either as activity H as measured approximately by pH meter or molar concentration H. 3 aq 2 HClaq CaCl. ANC is equivalent to alkalinity for samples without titratable particulate matter.
Surface water or soil water. What is a neutralization reaction. ANC is equivalent to alkalinity for samples without titratable particulate matter.
Prepare sulfuric acid titration solution. 𝐴 𝐶 𝑎 𝑖 𝑎 𝑖 𝑎 𝑎 𝑎 𝑖 MaterialsEquipment Funnel Beakers 250 mL 2 Buret with stand and clamp Erlenmeyer flask 125 mL 2 for filling buret Mortar and pestle Volumetric pipet 2000 mL. Acid Base Salt water.
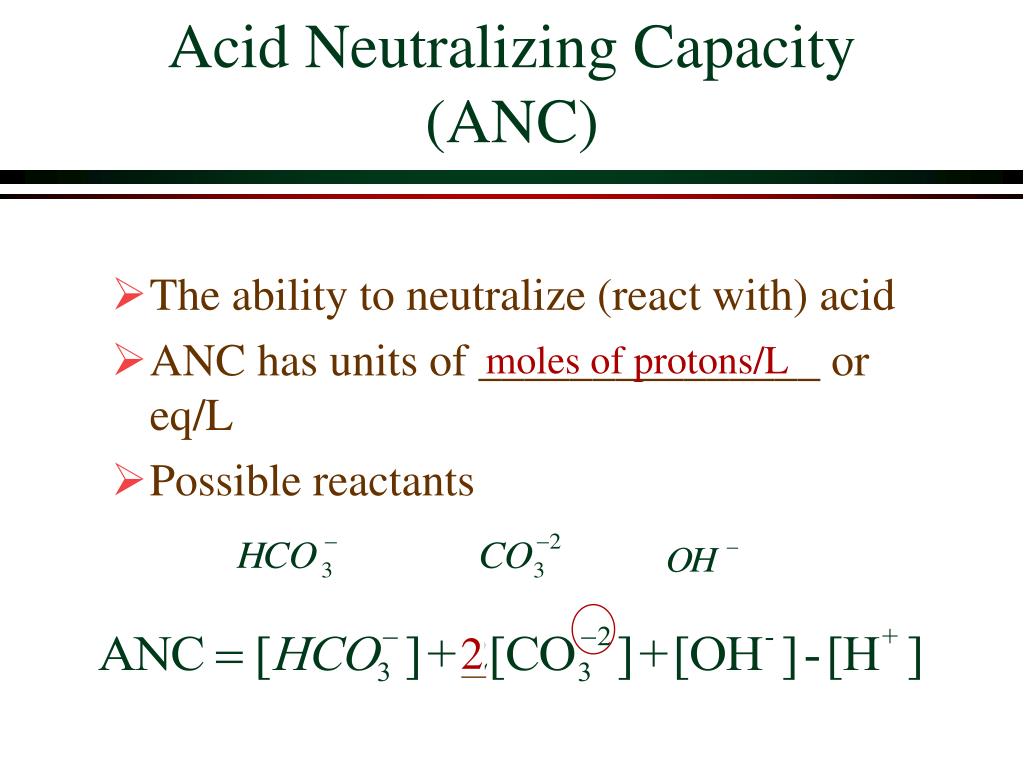
How do you calculate the neutralizing power of an antacid. The balanced equations for the neutralization of acid with these active ingredients are. ANC is the acid-neutralizing capacity of solutes plus particu-lates in an unfiltered water sample reported in equivalents per liter or milliequivalents or microequivalents per liter.
The overall reactions are. ANC is defined as the difference between cations of strong bases and anions of strong acids see below or dynamically as the amount of acid needed to change the pH value from the samples value to a chosen different. Volume molesMolarity Volume moles H 0075 Molarity moles H moles OH - Volume 0002 moles0075 Molarity Volume 00267 Liters Volume 267 milliliters of HCl Performing the Calculation 267 milliliters of 0075 M HCl is needed to neutralize 100 milliliters of 001 Molarity Ca OH2 solution.
Q2 Caclulate total number of moles of H in the beaker before neutralization by antacid-tablet. Geological Survey TWRI Book 9 498 10 ALK. The method is based on the fact that one gram equivalent of the acid completely neutralizes one gram equivalent of the base and vice-versa.
Introduction Acid neutralizing capacity ANC or alkalinity Alk of natural waters is widely measured as a key water quality parameter in both regional water quality surveys and in- tensive watershed studies. 2 aq H. Testing can determine how much acid would need to be added to a quantity of water to change the pH.
The choice only affects the slope of F 1 since H H γ. Add 05 mL concentrated H 2 SO 4 specific gravity 184 gmL to 950 mL DIW. PH is a measure of the hydrogen ion H.
4 Mg OH 2 2 HCl Mg 2 2 Cl 2 H 2 O CaCO 3 2 HCl Ca 2 2 Cl CO 2 g H 2 O Notice the 2-to-1 mole ratio of HCl-to-base. Calculate the number of mEq of acid consumed by the formula. Measurement of Acid Neutralizing Capacity CEE 4530.
Acid neutralizing capacity is a measurement of the buffering abilities in a sample of water.

Unit 13 Acids Bases Chemistry Chapter 19 Welcome To The Gowerhour Ppt Download
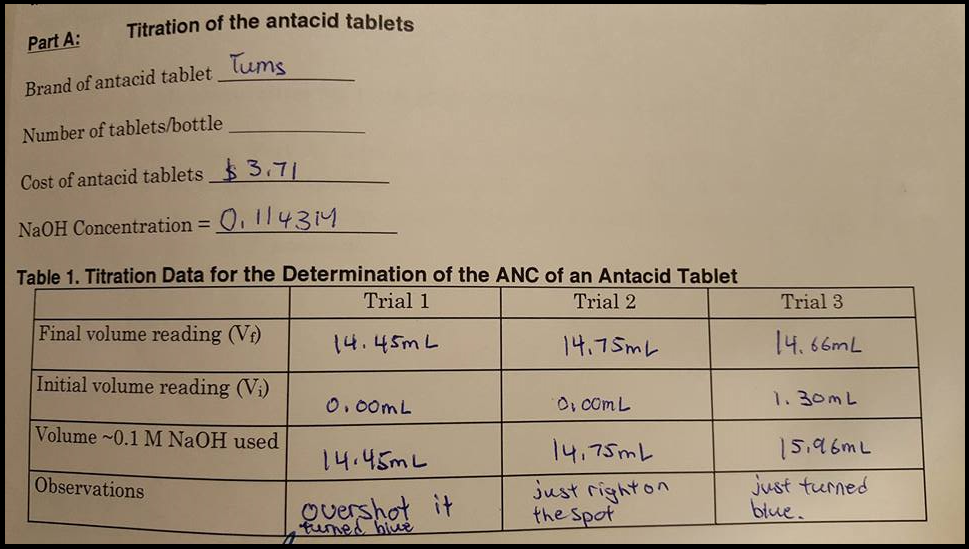
Acid Neutralizing Capacity Of Antacid Tablets Need Chegg Com

Acid Neutralizing Capacity Of An Antacid

Measurement Of Acid Neutralizing Capacity

Pdf Evaluation Of The Acid Neutralizing Capacity Of Some Commercially Available Brands Of Antacid Tablets In Nigeria
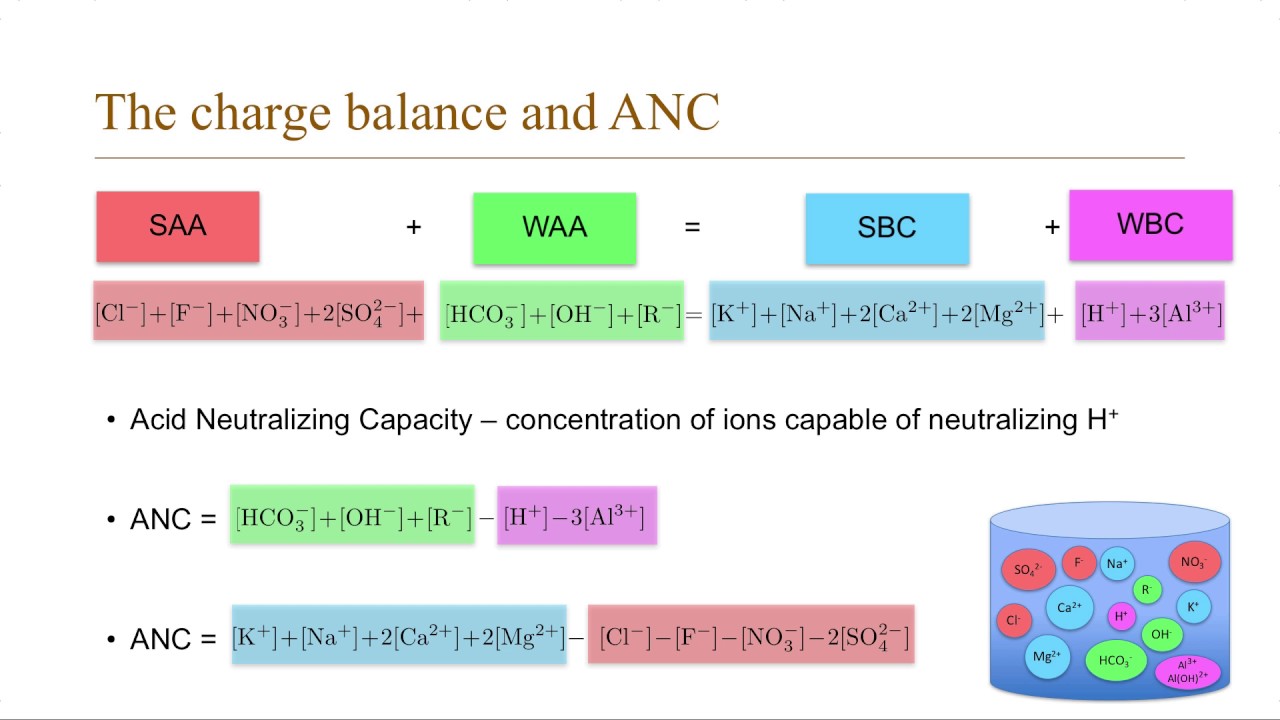
Water Chemistry 3 Charge Balance And Anc Youtube

Ph Acid Neutralizing Capacity Acid Rain Announcements Canoe Trip Canceled Due To High Water Discharge More Than Doubled Between Last Thursday And Sunday Ppt Download
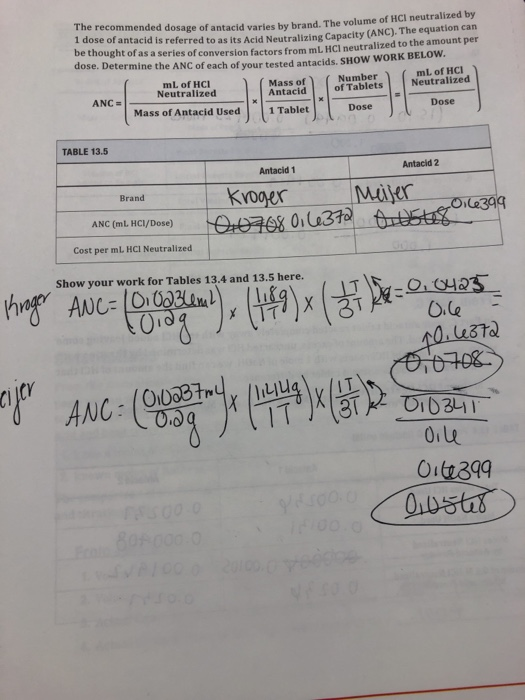
Solved Recommended Dosage Of Antacid Varies By Brand The Chegg Com
Ppt Acid Lake Remediation Powerpoint Presentation Free Download Id 588871
Comments
Post a Comment